Abstract
Background Hyperleukocytosis is observed in 5% to 20% of patients with newly diagnosed acute myeloid leukemia (AML) and is associated with an increased risk of early complications and mortality. While being used frequently in patients with AML and hyperleukocytosis, the clinical utility of leukapheresis has not been conclusive. Low-dose chemotherapy has also been used recently as a cytoreduction method in these patients, but the data are limited.
Objectives: To describe and compare the clinical and laboratory characteristics, early adverse events, and outcomes of children with newly diagnosed AML and hyperleukocytosis according to cytoreductive methods; leukapheresis, low dose chemotherapy (cytarabine), or no intervention.
Methods: We studied patients with newly diagnosed AML treated on three multi-institutional St. Jude protocols, AML97, AML02, and AML08, between 1997 and 2017. Hyperleukocytosis was defined as white blood cell (WBC) counts of 100 x 10 9/L or higher at diagnosis. The decision of cytoreductive treatment was made as the discretion of the treating physician. Leukoreduction was used in the AML97 and AML02 studies, and cytarabine (100mg/m 2/dose every 12 hours) was the first choice for AML08 study. We reviewed baseline clinical characteristics and laboratory data (complete blood cell counts [CBC], chemistries, coagulation) and adverse effects (grade 3 or higher on neurologic, renal, respiratory, and hemorrhagic complications based on Common Terminology Criteria for Adverse Events) from diagnosis to day 14 of protocol-based chemotherapy. Cairo-Bishop criteria was used for laboratory/clinical tumor lysis syndrome. The time from the first CBC to administration of protocol-based chemotherapy was calculated.
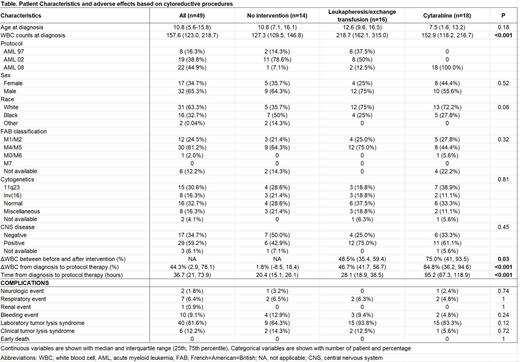
Results: A total of 49 patients were identified: 8 patients in AML97, 19 in AML02, and 22 in AML08) (Table). The age at diagnosis was 10.8 years with a median initial WBC count of 157.6 x 10 9/L; CNS (CNS 2, 3 or traumatic lumbar puncture with blasts) was seen in 29 (59.2%) cases. FAB M4 or M5 subtype was found in 30 patients (61.2%), 11q23 abnormalities in 15 (30.6%) and inv(16) in 8 (16.3%). In regards to leukoreduction method, 16 patients received leukapheresis (14 patients in AML97/02 and 2 in AML08), 18 cytarabine (all in AML08) and 1 hydroxyurea (in AML08); 14 did not receive leukoreduction (13 patients in AML97/02 and 1 in AML08). Leukapheresis was used more often in patients with higher diagnostic WBC counts (218.7 x 10 9/L) than those treated with cytarabine (152.9 x 10 9/L) or without intervention (127.3 x 10 9/L) (P<0.001). The decrease of WBC counts (%) before and after intervention was more pronounced among patients treated with cytarabine than those treated with leukapheresis (75% vs. 48.5%, P=0.03). When decreases in WBC counts were evaluated from the first CBC to the initiation of protocol therapy, cytarabine treatment was associated with more decreases in WBC counts from baseline (84.8%) than leukapheresis (46.7%) or no intervention (1.8%) (P<0.001). Patients who received cytarabine intervention had a longer median time from the first CBC to initiation of protocol therapy (95.2 hours) compared to those who received leukapheresis (28.1 hours) and no intervention (20.4 hours) (P<0.001). No early deaths were observed from the time of diagnosis to two weeks after initiation of protocol chemotherapy, and no statistically significant differences were noted in the incidences of neurologic, pulmonary, renal, hemorrhagic events, or laboratory/clinical tumor lysis syndrome among these three groups.
Conclusion: Low-dose cytarabine treatment appears to be a safe and effective mean of cytoreduction for patients with AML and hyperleukocytosis. Further studies are needed to determine if this approach is preferable among patients treated with contemporary treatment.
Pui: Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: Data Monitoring Committee.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal